 A student wanted to do some experiments to determine the specific heat of various substances. She constructed a calorimeter by using two metal cans, one nested inside the other, as shown in the diagram. A student wanted to do some experiments to determine the specific heat of various substances. She constructed a calorimeter by using two metal cans, one nested inside the other, as shown in the diagram.
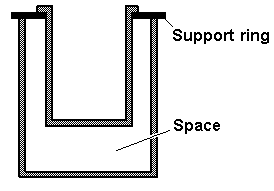
She plans to put water into the inner can and then add hot samples to this water to make the necessary measurements.
Of the following, the best material to use for the support ring is
- smooth aluminum.
- dull, black steel.
- rough copper.
- heavy cardboard.
- the same material as the can.
Explain why you chose your answer and why the other options were not chosen.
The space between the inner and outer cans should be filled with
- air.
- ice.
- water.
- a high density liquid.
- a low density liquid.
Explain why you chose your answer and why the other options were not chosen.
Which of the following would have to be known to obtain accurate results with this apparatus?
- The specific heat of the inner can.
- The specific gravity of the inner can.
- The mass of the inner can.
- 1 only
- 2 only
- 3 only
- 1 and 2
- 1 and 3
- 2 and 3
- 1, 2, ans 3
Explain how you arrived at your answer.
Source: Adapted from Handbook on Formative and Summative Evaluation of Student Learning by B. S. Bloom, J. T. Hastings, & G. F. Madaus, McGraw Hill, 1971, p599.
In the spring of 1996 five tank cars containing propane (molar mass = 44.09 g/mol) derailed in Weyauwega, WI forcing evacuation of the town for over a week. Residents within a square mile of the accident site were unable to return to their homes for over two weeks.
(a) Assuming that each tank car had a capacity of 33,500 gal, knowing that the density of liquid propane at its boiling point (-44.5°C) is 0.5853 g/cm3, and assuming that the propane vaporized and warmed to 25°C, how many kJ of heat would be evolved if all the propane reacted explosively with oxygen in the air?
| Substance |
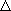 Hof Hof |
| C3 H8(g) |
-103.8 |
| CO2(g) |
-393.5 |
| H2O (l) |
-285.8 |
(b) 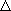 H for the explosive decomposition of TNT (C7H5N3O6) to water, nitrogen, carbon monoxide, and carbon is -1066.1 kJ/mol. How many kilotons (1 Ton=103 kg) of TNT would provide the amount of energy equivalent to that produced by the combustion of five tank cars of propane? H for the explosive decomposition of TNT (C7H5N3O6) to water, nitrogen, carbon monoxide, and carbon is -1066.1 kJ/mol. How many kilotons (1 Ton=103 kg) of TNT would provide the amount of energy equivalent to that produced by the combustion of five tank cars of propane?
[For comparison: The fission bombs dropped on Hiroshima and Nagasaki in 1945 each released the equivalent of about 20 kilotons of TNT. Modern fission weapons range from about 1 to 100 kilotons. Superbombs stockpiled by the USA and USSR are thought to be capable of delivering about 20 megatons of energy each.]
Source: Used with permission from Moore, J. W., Stanitski, C.L., Wood, J. L., Kotz, J. C., and Joesten, M. D., The Chemical World, 2nd ed., Saunders, Philadelphia, 1998.
Suppose you are a member of an environmental group and have been assigned to evaluate various ways of delivering milk to consumers with respect to free energy conservation. Think of all the ways that milk could be delivered, the kinds of containers that could be used, the ways they could be transported, and whether the containers could be reused (refillable) or recycled. Define the problem in terms of the kinds of information you would need to collect, how you would analyze the information, and the criteria you would use to decide which systems are more efficient in use of free energy. Do not try to collect the actual data you would use, but define the problem well enough so that someone could collect the necessary databased on your statement of the problem.
Source: Used with permission from Moore, J. W., Stanitski, C.L., Wood, J. L., Kotz, J. C., and Joesten, M. D., The Chemical World, 2nd ed., Saunders, Philadelphia, 1998.
|



