Bonding and Structure
128. (Liquid crystals, phase
changes) How many hydrogen atoms are present in the structure of the liquid
crystal shown below?

13, 17, 21
Which part of the molecule is more rigid?
left box, right box
Schematically, the solid liquid crystal has molecules aligned in a repeating
pattern. In the liquid, order is lost. In the liquid crystal, there is still
orientational order. Phase transitions are observed at 21 and 45 degrees
C.
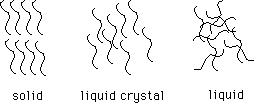
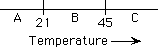
In which region would the liquid crystal be the stable phase?
A, B, C
136. (Coordination chemistry)
At-Seat Demonstration: Octahedral models and rubber bands are distributed,
and the rubber bands represent bidentate ligands. The formula for an octahedral
complex with one bidentate ethylenediamine (en) ligand is Ni(en)(H2O)x
with x =?
2, 4, 6
140. (Dipole moments, polarity)
Which of the following normal stretching modes for the carbon dioxide molecule
will cause a change in dipole moment and thus allow the molecule to absorb
infrared radiation?
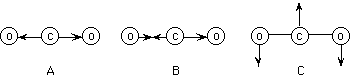
A, B, C
145. (Chirality) In experiment
A the d optical isomer of a chiral anion is added to equal concentrations
of the d and l isomers of a chiral cation. Only the d
isomer of the chiral cation precipitates out in the salt. If in experiment
B, the l isomer of the chiral anion is added, which of the cations
will precipitate out?
d isomer, l isomer, both
Demonstration: Use gloves and hands to show the mirror-image relationship:
151. (Acids and bases, Lewis
dot structure) Are more bonds needed to complete the Lewis dot structure
of acetic acid?
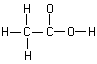
yes, no
155. (Density, equilibrium)
Demonstration: In beaker A, an ice cube of pure D2O is
to be placed in pure liquid H2O. In beaker B, an ice cube of
pure H2O is to be placed in pure liquid D2O. In which
of the two beakers will the ice cube sink?
A, B
159. (Diffraction, optical
transforms, layer sequences; Ch. 3 & 4 "Companion" Experiment
4) When looking down onto a face of the FCC and BCC cubic unit cells, the
two arrangements of atoms shown below are seen by superimposing layer sequences.
Which corresponds to the FCC structure?

A, B
Which X-ray diffraction pattern corresponds to the FCC structure?

A, B
183. It has been predicted that if we could put the element
hydrogen, normally an invisible gas of diatomic molecules at room temperature
and pressure, under sufficiently high pressure - millions of atmospheres
- it can become a metal!
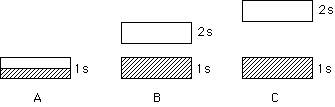
Which of the above three band diagrams represents solid hydrogen?
A, B, C
To conduct the experiment, the gas is compressed in a so-called anvil. The
anvil must be made of a material that is 1) strong enough to withstand high
pressures and 2) transparent, so that the sample can be viewed while it
is being squeezed. Which of the following materials is the best choice for
the anvil material?
diamond, NaCl, graphite
If you could conduct a diffraction experiment on the hydrogen sample while
it was being squeezed, what do you predict would happen to the spacing between
diffraction spots as the atoms are placed under increasing pressure?
the spacing would increase
the spacing would decrease
the spacing would stay the same
196.
How many chiral carbon centers are in the following molecule?
0, 1, 2, 3
219. (Companion, Ch. 2) Dipoles
can also occur in the solid state. If positive and negative charges are
distributed around a regular hexagon, as shown in Figure A, is there a net
dipole moment?
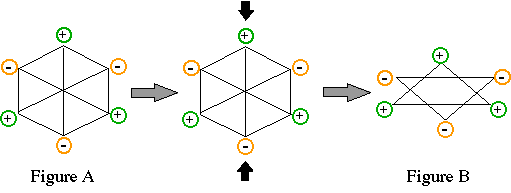
yes, no
If the hexagon is compressed in the vertical direction, as shown in Figure
B, is there a net dipole moment?
yes, no
225.
The unstable fulminate ion (CNO-) has two possible resonance structures.
Based on formal charge considerations, which is the better structure?
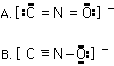
A, B
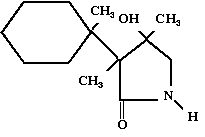
226.
In the organic molecule diagrammed below which carbon uses trigonal planar
geometry (or sp2 hybrid orbitals) in the bonding?
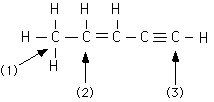
1, 2, 3
236. Dissecting a Polymerization Reaction
Polyethylene is formed when the molecule ethylene is polymerized. Approximately
how many C-C single bonds are formed from n molecules of ethylene? [The
instructor may want to draw approximately half a dozen H2C=CH2
molecules in a line, sketch electron movement with arrows, and draw the
resulting alkane to help students visualize this process.]
n
2n
3n
For the net reaction
nC2H4(g) ---> -[CH2-CH2]-n(s)
What is the sign of  S?
S?
 S>0
S>0
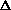 S<0
S<0
 S=0
S=0
Given that a mole of C=C double bonds is worth approximately 600 kJ/mol
and a mole of C-C single bonds is worth approximately 350 kJ/mol, what is
the sign of 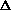 H?
H?
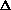 H>0
H>0
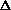 H<0
H<0
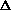 H=0
H=0
Does 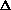 H or
H or 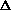 S drive this reaction?
S drive this reaction?
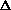 H
H
 S
S



More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..


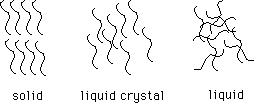








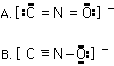
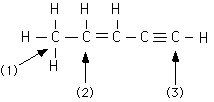
![]() S?
S?S>0
S<0
S=0
![]() H?
H?H>0
H<0
H=0
![]() H or
H or ![]() S drive this reaction?
S drive this reaction?H
S
![]()
![]()
![]()