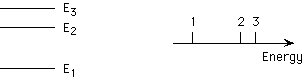
A, B
in the UV region at > 3 eV, in the visible between 1.7 and 3 eV,
in the IR at < 3 eV
1, 2, 3
A plot of these three emission bands is shown below. Which of the three
peaks corresponds to the transition from E3 to E1?
1, 2, 3
yes, no
Will they have the same intensity of color in a given pathlength?
yes, no

A, B, C, D
Sn
InSb
CdTe
InTe
donor, acceptor
Complete the equilibrium reaction shown below for this dopant:
![]()
The charge on the product ion is:
positive, negative
The ionization of N leads to the production of a(n):
electron, hole
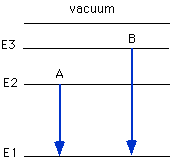
Which arrow shown below represents the electronic transition corresponding
to the ionization of N?

A, B
According to LeChatelier's Principle, adding boron (an acceptor) to N-doped
diamond will shift the equilibrium of equation 1 toward the:
products, reactants
(A similar set of questions could be asked about doping with B, which
can produce yellow diamonds, such as the Smithsonian
Canary Diamond.)
A, B

A, B, C
233. When intense red light strikes a sample of Cs metal in a photoelectric effect experiment, no electrons are ejected from the surface. In contrast, exciting the sample with weak blue light ejects electrons, whose kinetic energy can be measured.
These results are consistent with which of the following conclusions?
Red photons have more energy than blue photons.
Red photons have less energy than blue photons.
Red photons have the same energy as blue photons.
A plot of the kinetic energy of the ejected electron vs. incident photon
frequency, ![]() , would look like
, would look like
A.
B.
An equation describing this experiment is
235. (Nuclear chemistry, periodic properties) Researchers are trying to prepare element 114 (Science, 24 October 1997, 278, 571-572.). If a compound could be made from only this element and hydrogen, based on its position in the periodic table, how many H atoms would you expect to bond to element 114?
1, 3, 4, 5
239. Consider two LEDs, one red and one green, both emitting at their band gap energy. Which operating LED can cause a photovoltage in the other LED?
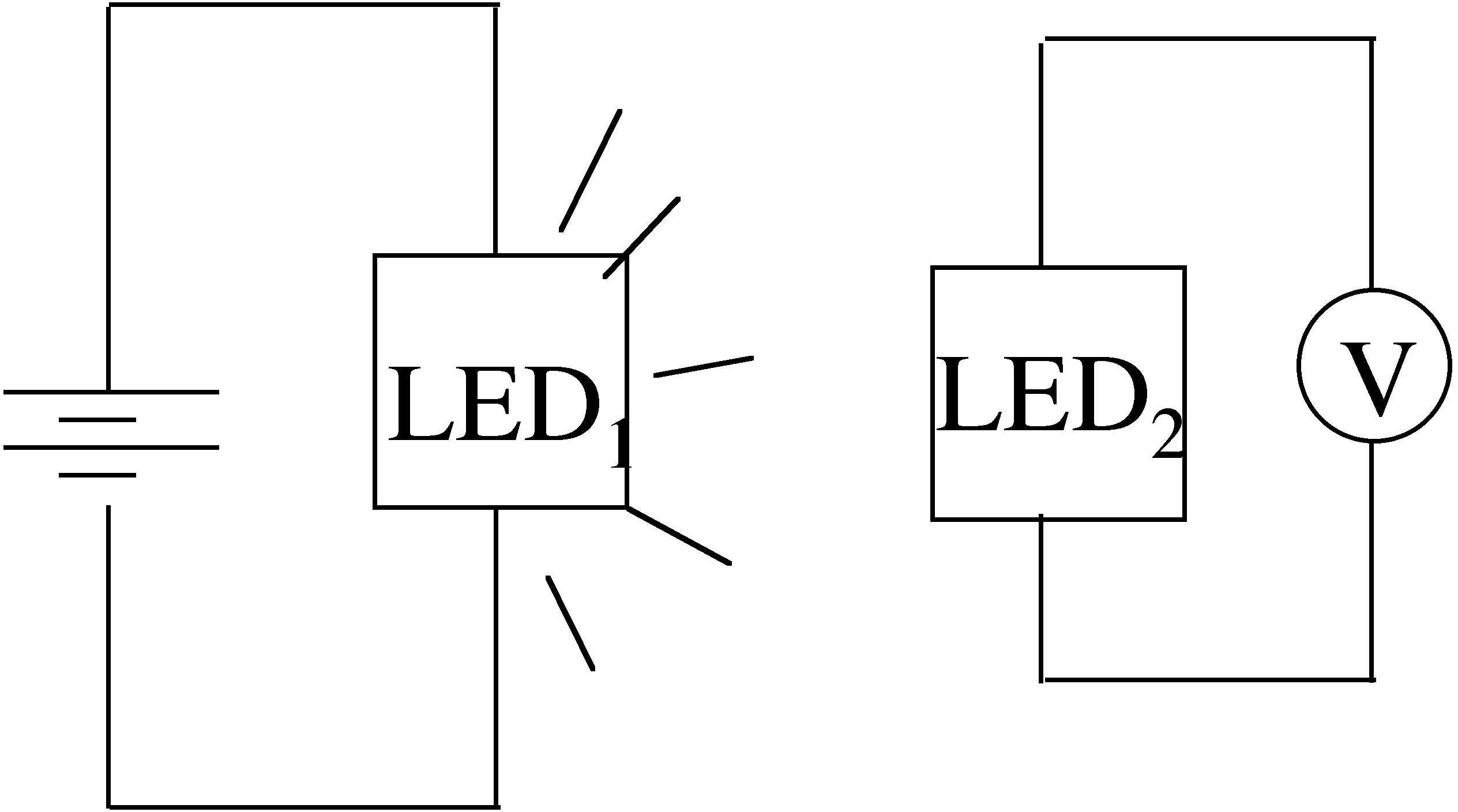
The red LED causes photovoltage in the green LED
The green LED causes photovoltage in the red LED
![]()
![]()
![]()
More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..