Equilibrium
166. For the reaction:
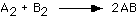
The concentration-time graph is as follows:

What is the value of the equilibrium constant?
1, 2, 4
167. The equilibrium constant expression
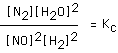
corresponds to which of the following balanced chemical reactions?
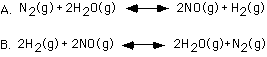
A, B
168. For the reaction

The relationship between Kc and Kp is
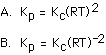
A, B
169. For the reaction
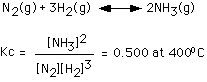
Suppose we make a mixture with the following concentrations:
[NH3] = 1.0M, [N2] = 1.0M, [H2] = 1.0M
In which direction will the reaction go?

A, B
170. In the equilibrium
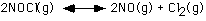
The initial conditions are:
[NOCl]o = 1.0 M
[NO] = 0.0 M
[Cl2] = 0.0 M
Which is the correct expression for the equilbrium concentrations?
A. [NOCl] = 1.0-x, [NO] = x, [Cl2] = x
B. [NOCl] = 1.0-2x, [NO] = 2x, [Cl2] = x
A, B
171. Which of the following equilibria
will move left (products ---> reactants) when the volume of the reaction
vessel is increased?

A, B, C
172. The equilibrium constant for
the reaction
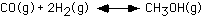
is 4.3 at 250oC and 1.8 at 275oC.
The reaction is
exothermic, endothermic
190. If the density of graphite
is about 2 g/mL and that of buckyball is about 1.5 g/mL, in which direction
would the application of high pressure drive the equilibrium?
toward formation of graphite, toward formation of buckyball, neither
would be formed preferentially
209. The equilibrium constant for autoionization of silicon
at 298 K is about 1020 cm-6. If the silicon is doped
with phosphorus to give an electron concentration, n, of 1020
cm-3, the concentration of valence band holes, p, is:
1020 cm-3
1015 cm-3
1010 cm-3
105 cm-3
211. The element Bi melts at 271 degrees C and has a density
of 9.73 g/ml as a solid and 10.05 g/ml as a liquid at this temperature.
For the equilibrium, Bi(s) <==> Bi(l), melting is favored in this
endothermic reaction by
increasing, decreasing temperature
or
increasing, decreasing pressure
217. Consider the gaseous equilibrium of H2 (g)
+ I2 (g) <==> 2 HI (g). As sketched below, the pressures,
P, of these three gases are at equilibrium in a container. At some time,
t, extra I2 is added, as shown. Which of the sets of curves shows
how the system will respond to this situation?
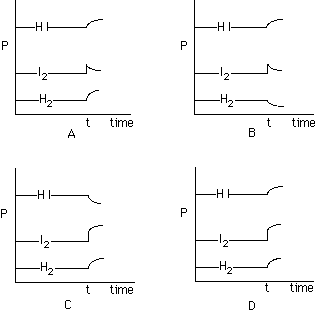
A, B, C, D



More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..
![]()

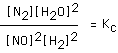
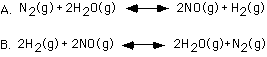
![]()
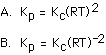
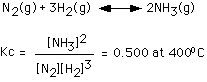
![]()
![]()

![]()
![]()
![]()
![]()
![]()