Other Topics
34. (Thermal conductivity; Ch. 7 "Companion")
If students have a seat/desk with metal and wooden parts that are not in
direct contact with their bodies, ask about the relative temperature: Which
is colder? Demonstration: Measure the temperature of each part with
a digital thermometer.
wood, metal, both are at the same temperature
(Tie in with thermal equilibrium and the calculated value of ambient
thermal energy as RT.)
If the temperature of the room goes from 20 degrees C to 40 degrees C, the
ambient thermal energy
doubles, is halved, increases by less than 10%
At-Seat Demonstration 7.5 "Companion": Touch the wood and
metal part of the desk. Which material conducts heat better and thus has
a higher thermal conductivity?
wood, metal, the two materials are the same
88. (Isotopes, Half-life) The half-life of 238U
is 4.5x109 years; that of 235U is 7.1x108
years. If at the moment of the birth of the universe there were equal amounts
of 238U and 235U , which is now in excess?
235U, 238U, still equal amounts
Referring to the graph below, which line represents the decay of 238U,
as opposed to that of 235U?
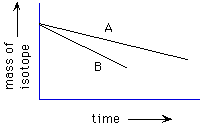
A, B
91. (Thermodynamics) Demonstration: Pour hot water
into a room temperature vessel. What is the equilibrium temperature of the
water?
> room temperature, = room temperature, < room temperature
108. (Radioactivity) Does t1/2 depend on chemical composition?
yes, no
110. (Thermodynamics, logarithms) Which plot represents
the temperature of a beaker of hot water left to sit out in a cool room?
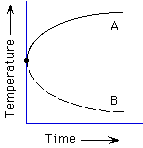
A, B
A semilog plot of log [(Temp. of hot water) - (Room temperature)] vs.
time is linear. Can the equilibrium temperature difference of zero be shown
on such a plot?
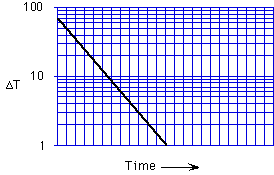
yes, no
111. (Logarithms) Omit some numbers from any of the indicated
logarithmic number lines and ask what they are.
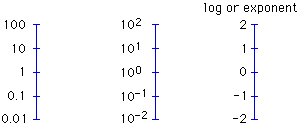
113. (Thermal energy, work hardening; Ch. 6 "Companion")
Demonstration 6.4 "Companion": After work hardening a piece
of copper metal, it can be made soft again by
heating, cooling
114. (Plastic deformation, slip planes; Ch. 6 "Companion")
Which situation allows easiest slippage of planes of atoms past one another,
as sketched below?
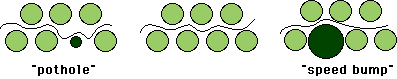
identical spheres in both layers, a layer with a large impurity atom,
a layer with a small impurity atom
Which of the two impurities acts like a "speed bump" and which
like a "pothole," in impeding movement along slip planes?
125. (Chromatography) Demonstration: Simultaneously
drop marbles and ping pong balls down the ICE model chromatography column.
Which balls will get to the bottom first?
marbles, ping pong balls
126. (Chromatography, equilibrium) The chromatographic
equilibrium for a species A can be described as A in mobile phase <==>
A in stationary phase.
A mixture of two volatile compounds is injected onto a column with air,
which doesn't interact with the column. Which of the three peaks below is
air?
1, 2, 3
Which of the other two peaks corresponds to the larger equilibrium constant
K?
1, 2, 3
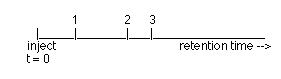
130. (Nuclear chemistry) Can a chemical reaction transform
lead to gold?
yes, no
131. (Nuclear chemistry) A proton would be denoted
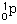 ,
,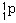
132. (Nuclear chemistry, half lives) From the semilog
plot of log(disintegration rate) vs. time (below), the approximate half
life of 14C is
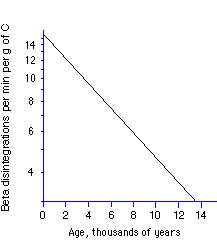
2000 years, 6000 years, 10,000 years
133. (Nuclear chemistry, critical mass) Consider a small
sphere A and a larger sphere B. Which has a larger surface-to-volume ratio?
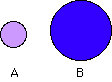
A, B
In order to sustain a nuclear chain reaction, is a large or small surface-to-volume
ratio desired?
large, small (large sphere having at least the so-called critical mass)
134. (Nuclear chemistry) From the plot of binding energy
as a function of nucleon, more stable nuclei are made by
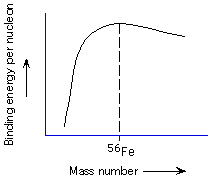
moving toward the maximum in the plot at 56Fe, moving away
from the maximum in the plot at 56Fe
Which direction is fusion of nuclei and which is fission (see plot below)?
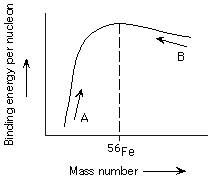
A is fusion and B is fission, A is fission and B is fusion




More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..




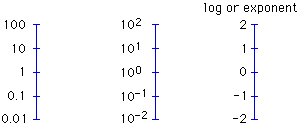

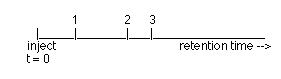
,

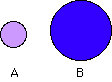


![]()
![]()
![]()