![]()
![]()
A.  B.
B.  C.
C.  D.
D. 
A.  B.
B.  C.
C. 
D. 
A.  B.
B.  C.
C.  D.
D. 
A.  B.
B. 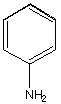 C.
C. 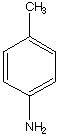 D.
D. 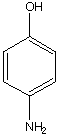
1092. Which compound has the highest boiling point?
CH4, H2O, NaCl
Which compound has the lowest boiling point?
CH4, H2O, NaCl
1093. Which compound has the highest boiling point?
CH4, CH3OH, CH3CH3
Which compound has the lowest boiling point?
CH4, CH3OH, CH3CH3
1094. Which compound has the highest boiling point?
A. pentane
B.hexane
C.2-methylbutane
Which compound has the lowest boiling point?
A. pentane
B.hexane
C.2-methylbutane
a. Which will have the higher boiling point?
A. CH3Br
B. CH4
b. Which will have the lower boiling point?
A. CH3F
B. CH4
![]()
![]()
![]()
More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..