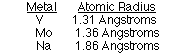
increase, decrease, stay the same
What will the freezing point do when lots of salt is dissolved in the
water?
increase, decrease, stay the same
increased by a factor of 10, decreased by a factor of 10, unchanged
The concentration of NaOH is
increased by a factor of 10, decreased by a factor of 10, unchanged
yes no
Will they have the same intensity of color in a given pathlength?
yes no
2, 3, 4
Which two metals are most likely to form a solid solution?
V and Mo, V and Na, Mo and Na
Additional example:

Which two elements are most likely to form a solid solution?
C and Si, C and Ge, Si and Ge
~10 mL, ~100 mL
How much water would need to be added to raise the pH to 3?
~10 mL, ~100 mL, ~1000 mL
Could you get a pH of 10 by continuing to add more water?
yes, no
increases by 50%, decreases by 50%, doesn't change
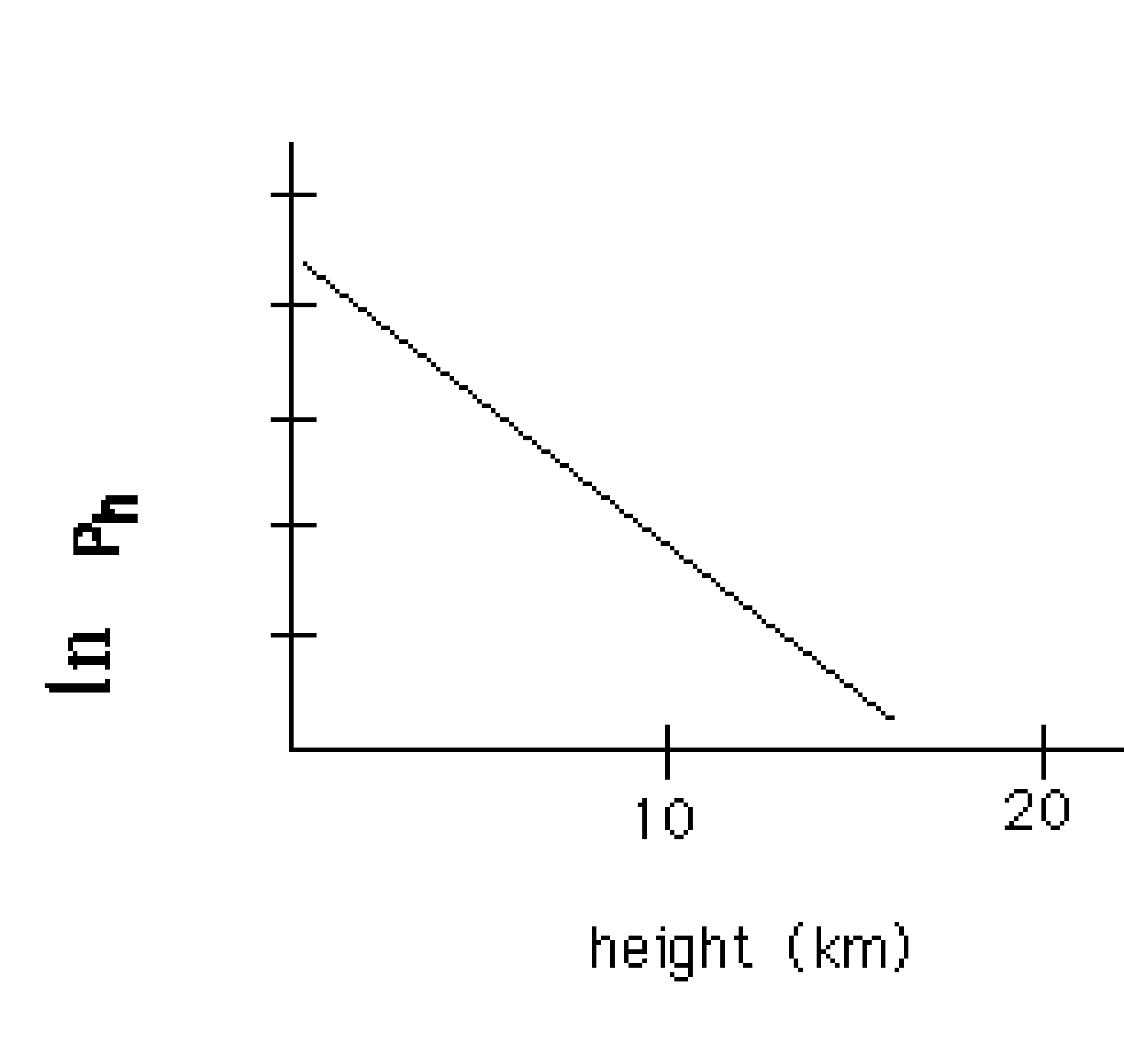
What effect will increasing the temperature have on the slope of the
line plotted above?
increase slope, decrease slope, the slope will stay the same
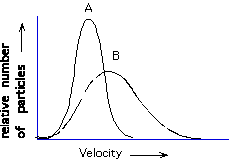
A, B
The kinetic molecular theory assumes negligible volume for the gaseous species
and no interactions among them. Comparing helium to a free electron gas
in a semiconductor, which seems better matched to the assumption that the
gas takes up no volume?
free electron gas, helium
Which of the above three band diagrams represents solid hydrogen?
A, B, C
To conduct the experiment, the gas is compressed in a so-called anvil. The
anvil must be made of a material that is 1) strong enough to withstand high
pressures and 2) transparent, so that the sample can be viewed while it
is being squeezed. Which of the following materials is the best choice for
the anvil material?
diamond, NaCl, graphite
If you could conduct a diffraction experiment on the hydrogen sample while
it was being squeezed, what do you predict would happen to the spacing between
diffraction spots as the atoms are placed under increasing pressure?
the spacing would increase
the spacing would decrease
the spacing would stay the same
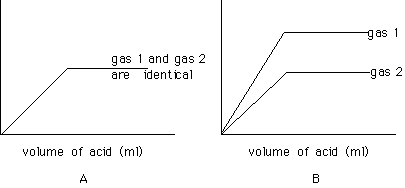
plot A represents the volumes of the two gases (H2S and H2Se) and plot B represents the masses of the two gases
plot A represents masses of the two gases and plot B represents volume of the two gases
Which of the two curves in plot B represents the mass of H2S?
1, 2
In which part of either plot is the acid the limiting reagent?
flat part
rising part
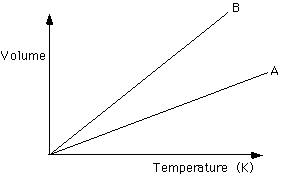
A, B
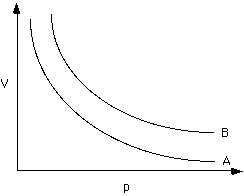
A, B
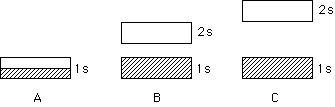
240. DEMONSTRATION Two samples of liquid Br2 are placed in each of two ampules that are then sealed. One ampule will be dropped so as to break in a transparent tube filled with air. The other will be dropped so as to break in an evacuated tube. Compare the relative rates of diffusion, which are made visible as the red-brown Br2 vapor fills each tube. The rate of diffusion will be:
The same in both tubes
Faster in the air-filled tube
Faster in the evacuated tube
![]()
![]()
![]()
More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..