|
Home
Table of Contents
Keyword Index
Textbook X-ref
Search
About CCA!
|
 |
| pH of Acetic Acid, Monochloro-, Dichloro-, Trichloro-, and Trifluoroacetic Acids |
 |

View Slide Thumbnails |
|

View Next Movie |
|
Discussion
The effect of substituting electronegative halogen atoms for the a-hydrogen atoms in acetic acid is demonstrated by comparing the pH of 0.1M solution of acetic acid, monochloro-, dichloro-, trichloro-, and trifluoroacetic acids. The pH differences are also shown by methyl violet indicator.
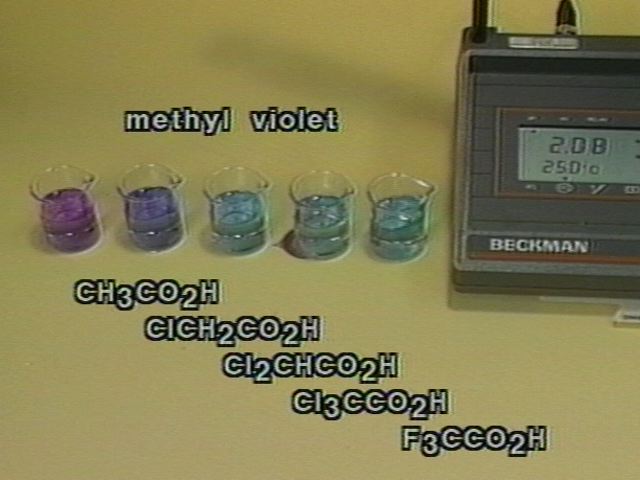
pH Differences as Shown by Methyl Violet Indicator |
|
|
|
|
|
Design, Text and Demonstrator:
|
|
| |
Gary Trammell
|
University of Illinois at Springfield, Springfield, IL 62794
|
|
Videographer/Editor:
|
|
| |
Steve Dykema
|
University of Illinois at Springfield, Springfield, IL 62794
|
|
Voice:
|
|
| |
Margaret Biddle
|
University of Wisconsin - Madison, Madison, WI 53706
|
|
Audio Production:
|
|
| |
Greg Minix
|
University of Wisconsin - Madison, College of Engineering, Madison, WI 53706
|
| |
Jerrold J. Jacobsen
|
University of Wisconsin - Madison, Madison, WI 53706
|
|

