Example 2 investigates the reaction of chlorine and hydrogen. This example illustrates a potential pitfall to the application the M.O. reaction prediction method. The HOMO/LUMO graphic and energy differences suggest that a reaction between these molecules is very unlikely. However, the reaction between these molecules is well known and produces HCl. The reason for this contradiction is that the reaction does not work as a single step bimolecular process. It has been shown experimentally that the reaction takes place through a multi-step mechanism. The first step is the photochemical decomposition of diatomic chlorine into atoms. In the second step, a single chlorine atom reacts with a diatomic hydrogen to initiate a chain reaction in which the product is formed.
Diagrams
This is an example of a reaction for which the use of the M.O. reaction prediction method is inappropriate.
Looking at the ball and stick models, the side-by side interaction of hydrogen and chlorine is a reasonable possibility. This could be achieved by a simple bimolecular, four-center collision.
Molecular Orbitals
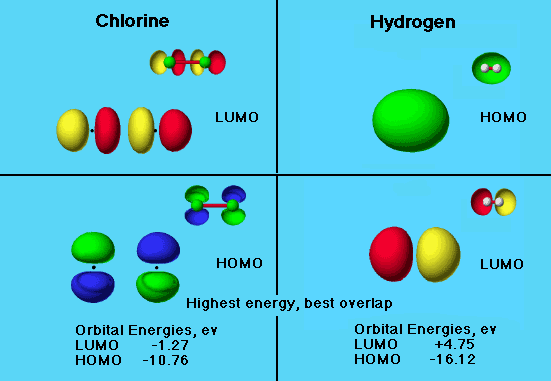
The graphic shows the MO’s as seen using the Compare feature in the database.
A check for the smaller energy differences of these orbitals is needed. Find the energy difference between LUMO of one and the HOMO of the other and vice versa.
Chlorine Hydrogen
LUMO -1.27 +4.75
HOMO -10.76 -16.12
LUMO (H€) – HOMO (Cl€) = +4.75 – (-10.76) = 15.51.
LUMO (Cl€) – HOMO (H€) = -1.27 – (-16.12) = 14.85.
The lower energy difference represents the most favorable interaction. Based on the calculation shown above, we would use the LUMO of Cl€ and the HOMO of H€. However, examination of the orbitals shows that there is no constructive overlap if the molecules are side by side. Unfavorable orbital overlap prevents sharing of electrons between the HOMO of H€ and the LUMO of Cl€.
The LUMO (H€) / HOMO (Cl€) combination is unfavorable because of the higher energy difference, but constructive overlap of the orbitals is possible. In this reaction electrons would be transferred from the chlorine HOMO and shared by the LUMO of hydrogen. Since chlorine is the more electronegative atom, such a transfer is unlikely.
Prediction
This graphic shows the MO’s with their front surfaces cut away to reveal the position of the ball and stick models for the mechanism proposed. Notice that the LUMO of the hydrogen molecule overlaps constructively with the HOMO of the chlorine molecule.
Although this combination has the higher energy difference, it is the only possible overlap for HOMO’s and LUMO’s of these two molecules.
In this proposed reaction electrons would be transferred from the chlorine HOMO and shared by the LUMO of hydrogen. Because chlorine atoms are much more electronegative than hydrogen atoms, such a transfer is unlikely.
Considering all of the information gathered by examination of the MO’s of these two molecules, the best prediction appears to be that they do not readily react with one another. However, experimentally the reaction of Cl€ and H€ to produce HCl is well known. The solution to this dilemma is that the reaction is not the simple bimolecular process illustrated. It has a multi-step mechanism in which the photochemical decomposition of diatomic chlorine into atoms is the first step. In the second step, a chlorine atom reacts with a hydrogen molecule to initiate a chain reaction. Our molecular orbital prediction model fails because the interaction that produces product does not occur between the two molecules—neither the Cl HOMO or LUMO is involved.
