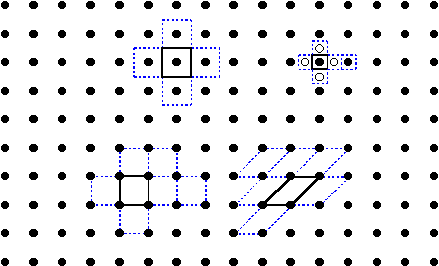
A unit cell is a building block of a crystalline solid.Shifting the unit cell along any of its edges by the length of the edge will generate identical cells to build up the entire crystal.If not chosen properly, shifts of the cell will not give an identical cell (upper right unit cell).Unit cells do not need to be squares or rectangles (lower right unit cell).Unit cells facilitate the counting of atoms comprising the solid (empirical formula).
|