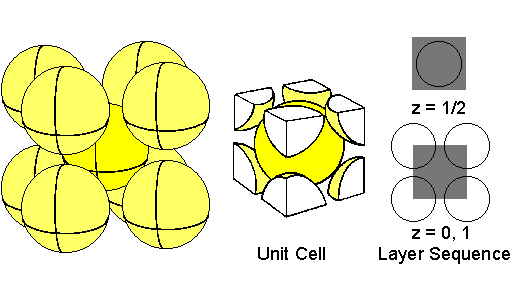Atoms centered at the corners of the unit cell and at the center of the cube.The atom in the center of the cube is entirely within the unit cell.The 8 atoms at the 8 corners are each 1/8 within the unit cell, and thus contribute collectively 1 atom to the unit cell.Therefore, the body-centered cubic (BCC) structure has 2 atoms per unit cell.The second layer has the center of the atom within the unit cell at an elevation on the z axis of 1/2 the unit cell length.
|