
In a real crystal, the coordination geometry about the cation is three- dimensional. The three common coordination geometries found in crystals of simple ionic compounds are illustrated on this page. Cations are represented by the smaller, blue spheres.
Click the links on the right to expose the cations in the 3 cases. You may view a definition of the underlined text above by clicking on it.
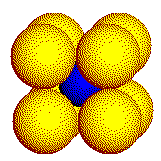
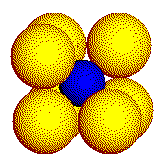
Large
cations touch eight or more anions. These 8 anions form a cube.
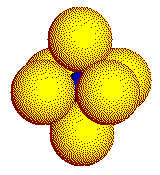
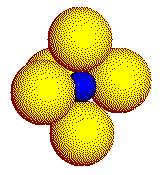
Mid-sized
cations touch six anions in an octahedron.

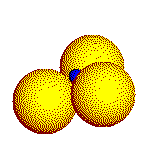
Small
cations touch four anions in a tetrahedron.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software