
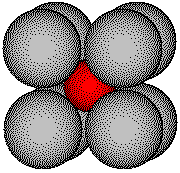
Simple ionic solids usually have more complicated structures than metals. The structure of a metal is usually determined by the packing of spheres of the same size. The structure of a simple ionic compound is usually determined by the packing of spheres of differing sizes.
For example:
Cesium metal:
cesium atomic
radius: 267 pm
Cesium iodide:
cesium ionic
radius: 169 pm
iodide ionic radius: 219 pm
The underlined text above links to definitions.
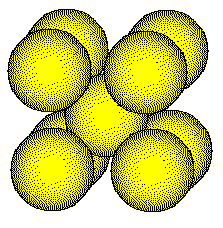
Cesium Metal
Cesium Iodide
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software