
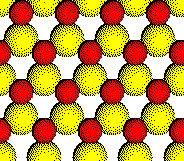
In compounds such as Li2S and K2O that crystallize with cations in tetrahedral holes in a close packed array of anions, the cations fill all of the tetrahedral holes in the array of anions.
An arrangement that fills half of the holes is found in the structures of CuCl, ZnS, and InP. One set of tetrahedral holes is filled and the other set is empty.
Please click the links on the right to view the tetrahedral holes either completely or half filled.
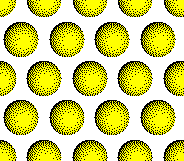
Anion
Layer 1

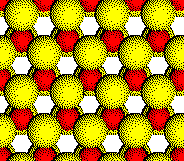
Anion
Layer 2
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software