Ionic
Radii:
Ca2+, 99 pm
F -, 136 pm
Radius Ratio = 99/136 = 0.73
The radius ratio is on the borderline between octahedral and cubic coordination. Experiment shows that Ca2+ ions occupy cubic holes.
The stoichiometry (one Ca2+ ion per two F - ions) requires that half of the cubic holes be occupied. In fact, the CaF2 structure consists of a simple cubic array of F - ions with Ca2+ ions filling half of the cubic holes.
Click on the image at right to view the Calcium Fluoride structure. The links in the text above go to definitions.
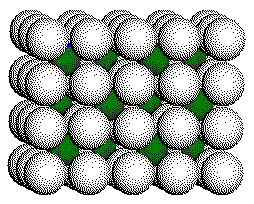
F
- Layer
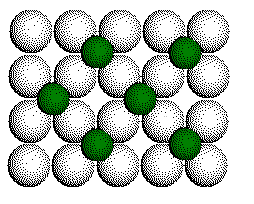
Ca2+
layer with one half of the cubic holes filled
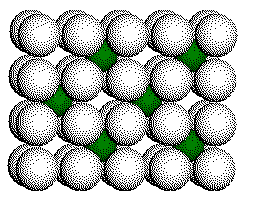
F
- Layer
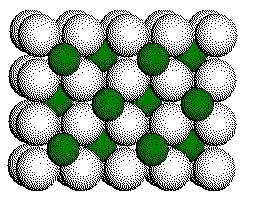
2nd
Ca2+ layer with one half of the cubic holes filled
F
- Layer
The
CaF2 structure consists of a simple cubic array of anions with
half of the cubic holes filled with cations.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software