
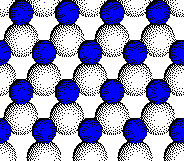
This is the first layer of the ZnS structure. To see the second layer click on the picture.
Click the links on the right, and follow the instructions that will appear below to review.
As a quick test, click on a location where the next
sulfer anion would appear in the third layer of ZnS.
This concludes the Structures of Ionic Solids book. Clicking any of the forward buttons or the Table of Contents button will return you to the main index page.
.gif)
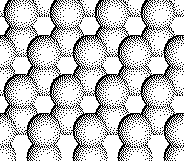

Sorry,
try again.
That is correct. A sulfur anion
would fill the chosen position.
To see the location of all of
the sulfur anions, click the
link labeled "fill" .
Note
that the first layer of zinc ions is covered by the second layer of sulfur
ions.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software