
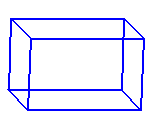
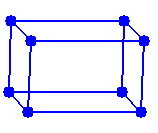
The unit cell of rutile and of structures isomorphous with rutile are tetragonal. In a tetragonal cell one cell edge differs from the other two. In the rutile unit cell the third cell edge is shorter. Lattice points are located on the corners of the unit cell.
Please click the links on the right to illustrate the above topics. The underlined text above links to a definition.
Rutile crystallizes with a tetragonal unit cell: the
a and b axes are of equal length, but are different from the c axis.
Rutile
crystallizes with a tetragonal unit cell.
Lattice points are located on the corners of the unit cell.
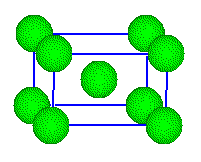
Titanium
ions are located on lattice points and in the center of the cell. The
unit cell looks body centered, but it isn't. The oxide ions are not arranged
in a body-centered array.
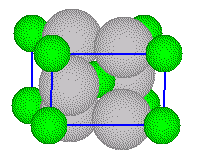
Two
oxide ions are located on the top face of the unit cell and two are located
on the bottom face, and two are located inside the cell.
The
titanium ions are located in one-half of the octahedral holes in the array
of oxide ions.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software