
The zinc blende unit cell contains four cations. Each is completely within the unit cell.
The zinc blende unit cell contains four anions in a face-centered cubic array. These are shared with several other cells.
A cubic ZnS unit cell contains 4 ZnS formula units.
Use the links on the right to illustrate the topics given above.


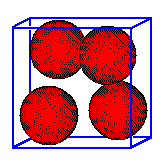
Each Zn2+ ion is inside the cell,
giving
4 Zn2+ ions per cell.
4 Zn2+ ions per cell.
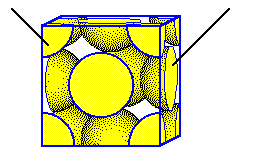
Sulfide ions are shared among several unit cells.
One eighth of a S2- ion at each corner is located inside the unit cell
One
half of a S2- ion in each face is located inside the unit cell
1/2 of a S2- ion in each face x 6 faces +
1/8 of a S2- ion at each corner x 8 corners
= 4 S2- ions per cell
1/8 of a S2- ion at each corner x 8 corners
= 4 S2- ions per cell
Each unit cell contains
4 Zn2+ ions and 4 S2- ions
4 Zn2+ ions and 4 S2- ions
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software