
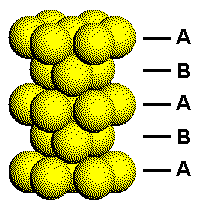
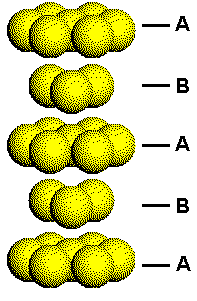
This figure shows a portion of a hexagonal closest packed structure with the layers arbitrarily labeled A and B. Each A layer has atoms with the same orientation. Each B layer has the same orientation, but this orientation is different from that of the A layers.
The difference in the orientation of the two types of planes in the HCP structure can be seen by considering how planes of close packed atoms stack to form three-dimensional solids.
Click the link on the right to either separate or put the HCP layers back together.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software