
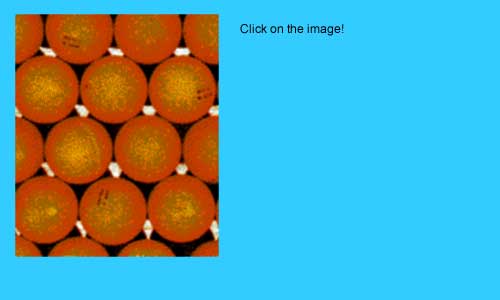
A layer of orange spheres sits in the dimples on a layer of dark spheres. Because only one-half of the triangular dimples in the first layer can be covered by spheres in the second layer, half of the triangular dimples are still visible.
A hexagonal closest packed structure results when atoms in the third layer sit directly above atoms in the first layer.
Click on a triangular dimple in the second layer (the orange layer of spheres) that will hold an atom in a HCP structure.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software