
Polonium is the only simple cubic metal. It crystallizes in a simple cubic space lattice with an atom located at each point.
The unit cell commonly selected for a simple cubic metal is a cube because this is the simplest repeating part of the lattice. The edges of the unit cells are of equal length and the cell angles are 90 degrees.
Additional information on the unit cell is provided on the next page.
Click the links on the right to illustrate. The underlined words in the text have definitions when clicked.

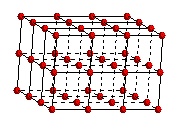
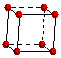
Simple
Cubic Space Lattice
Unit
Cell of Lattice
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software