 The figure represents a portion of an equilibrium mixture of two compounds related by the reaction 2A = B. The figure represents a portion of an equilibrium mixture of two compounds related by the reaction 2A = B.

Which of the following must be true of this equilibrium?
(a) K > 1
(b) K = 1
(c) K < 1
(d) K = 0
(e) K < 0
Even though both NH3 and C6H5NH2 are weak bases, NH3 is a much stronger base than C6H5NH2. Which of the following is most nearly correct at equilibrium for a solution that is initially 0.10 M in NH3 and 0.10 M in C6H5NH2?
(a) [OH-] = [NH4+]
(b) [NH4+] = [C6H5NH3+]
(c) [OH-] = [C6H5NH3+]
(d) [NH3] = [C6H5NH2]
(e) Both a and b are correct.
The odor of some household cleansers is due to the presence of ammonia, NH3, a weak base. List, in order of lowest concentration to highest concentration, all of the ionic and molecular species present in a 0.5 M solution of ammonia in water.
(i) Methanol can be prepared from water gas and additional hydrogen at high temperature and pressure in the presence of a suitable catalyst. Write the expression for the equilibrium constant for the reversible reaction.
2 H2(g) + CO(g) 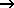 CH3OH(g) CH3OH(g)  H = -90.2 kJ H = -90.2 kJ
(ii) If a mixture of H2, CO, and CH3OH is at equilibrium, how will the concentrations of H2, CO, and CH3OH at a new equilibrium differ from their original concentrations when
a. more H2 is added?
b. CO is removed?
c. CH3OH is added?
d. the pressure on the system is increased?
e. the temperature of the system is increased?
f. more catalyst is added?
(Note: Part i is not a conceptual question. Part ii is, it asks for differences in concentrations, not for the directions of shift.)
The contribution of water to the concentration of H3O+ in a 0.10 M solution of acetic acid can be neglected when calculating the concentration of hydronium ion. Explain why we cannot neglect the contribution of water when calculating the concentration of hydroxide ion.
Which ion has the smallest value of Ksp for the reaction
MS(s) 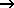 M2+(aq) + S2-(aq) M2+(aq) + S2-(aq)
(a) Hg2+ (a qualitative analysis group II ion)
(b) Ni2+ (a qualitative analysis group III ion)
(c) Ba2+ (a qualitative analysis group IV ion)
(d) All of these have the same value because Q = [M2+][S2-].
Which of the following 0.10 M solutions in water freezes at the lowest temperature? Assume the molarity and molarity are equal in these solutions.
(a) Acetic acid (Ka = 1.8 x 10-5)
(b) Boric acid (Ka = 5.8 x 10-10)
(c) Ammonia (K = 1.8 x 10-5)
(d) Methylamine (Kb = 4.4 x 10-4)
(e) Impossible to determine from the information given.
|



