|
Original question: The following data were obtained for the reaction
2 NO + O2 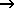 2 NO2 2 NO2
| [NO], M |
[O2], M |
Initial rate, M s-1 |
| 1 x 10-3 |
1 x 10-3 |
2.0 x 10-5 |
| 2 x 10-3 |
1 x 10-3 |
8.0 x 10-5 |
| 3 x 10-3 |
1 x 10-3 |
18.0 x 10-5 |
| 2 x 10-3 |
2 x 10-3 |
16.0 x 10-5 |
| 2 x 10-3 |
3 x 10-3 |
24.0 x 10-5 |
Which is the correct rate law?
- Rate = k[NO][O2]
- Rate = k[NO][O2]2
- Rate = k[NO]2[O2]
- Rate = k[NO]2
- Rate = k[NO]2[O2]2
Modified question: Which will have no effect on the rate of the reaction
CO(g) + NO2(g) 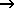 CO2(g) + NO(g) CO2(g) + NO(g)
if the rate law for the reaction is Rate = k[NO2]2?
- Decreasing the pressure of NO2
- Increasing the concentration of CO
- Increasing the temperature
- Adding a catalyst
- All of these will effect the rate.
|



