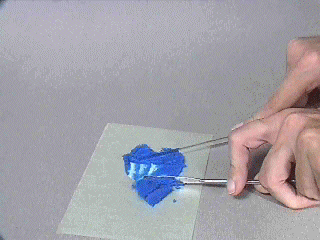
IMAGE. Spreading the solid out to allow it to dry.
|
The solid can be dried quickly by rinsing the solid with a volatile liquid (methanol, acetone, or ether are common) that the solid will not dissolve in. If the filtrate is to be collected, change the receiving flask before rinsing.
Once rinsed, let the solid stand until the solid is dry or remove the filter paper from the funnel spread the solid out on a clean, flat surface.
Sometimes the solid can be dried in an oven. However, discuss the risks of heating a sample in the oven with your instructor before placing any sample in an oven.
 A student is attempting to determine the %yield for an aspirin experiment. After collecting the solid by filtration, she records the following in her notebook:
A student is attempting to determine the %yield for an aspirin experiment. After collecting the solid by filtration, she records the following in her notebook:
| mass (g) |
time |
| 1.276 |
1:15 pm |
| 1.274 |
1:20 pm |
| 1.275 |
1:25 pm |
Is the sample dry?
Answer
Since three successive measurements are virtually identical, the sample appears to be dry. What should she record as the mass of aspirin recovered? Answer
She should record the average value for the last three measurements: 1.275 g. |