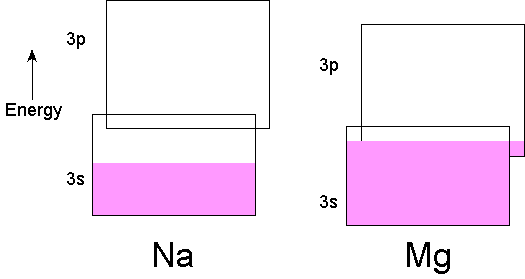
Metallic conductivity arises from a partially filled band.In Na there is one electron in the 3s orbital of each atom, a half-filled orbital. The 3s band is accordingly half-filled, since each orbital in the band can hold two electrons.In Mg there are two electrons in the 3s orbital of each atom. In the extended solid some of the 3p band overlaps the 3s band in energy. This results in an unfilled band and metallic conductivity.
|