
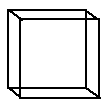
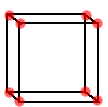
The unit cell of CsCl is a simple cubic. Lattice points are located only at the corners of the cell.
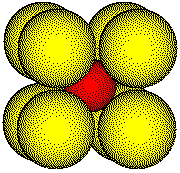
Chloride ions are located on the lattice points of the unit cell commonly used to describe the CsCl structure.
A cesium ion is located at the center of the cube.
Because the atom at the center of the cell is different from the atoms at the corners, the unit cell is simple cubic, not body-centered.
Click the links on the right in order to illustrate the topics listed above. Clicking the underlined text will link to a definition.
CsCl
crystallizes with a simple cubic unit cell
Lattice
points are located on the corners of the unit cell
Chloride
ions are located on the lattice points on the corners of the unit cell.
A
cesium ion is located at the center of the unit cell.

Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software