
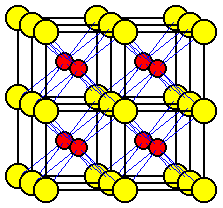
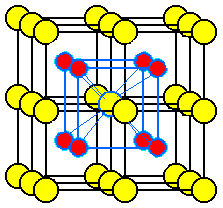
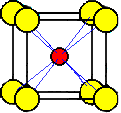
The unit cell most commonly selected for cesium chloride is a cube with a chloride ion at each corner and a cesium ion in the center of the cube.
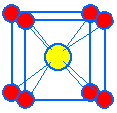
An alternate unit cell may be selected with cesium ions at the corners and a chloride ion in the center of the cube.
Click the links on the right to show an alternate unit
cell. The underlined link above goes to a definition.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software