
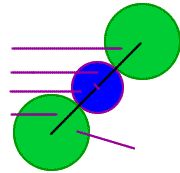
The length of the body diagonal is equal to two radii of the Cs+ ion plus two radii of the Cl- ion.
From the radius of one ion and the length of the body diagonal, we can find the radius of the other ion.
Click the links on the right in order to illustrate these topics. The underlined word above links to a definiton.
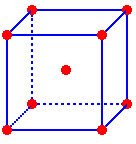




Body
Diagonals
in the CsCl Unit Cell
in the CsCl Unit Cell
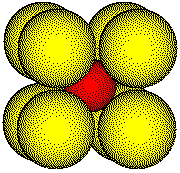
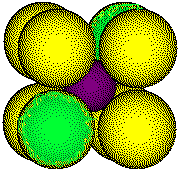
Body
Diagonal
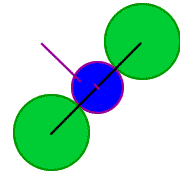
Outline of three atoms along the body diagonal
rCs+
is the
radius of
the Cs+ ion
radius of
the Cs+ ion
The length of the diagonal is 2 rCl- + 2 rCs+,
or, d = 2 [rCl- + rCs+]
rCl-
+ rCs+
+ rCs+
+ rCl-
rCl- is the
radius of
the Cl- ion
Body
Diagonal
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software