
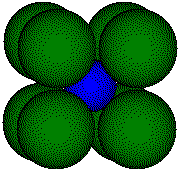
If we know the radii of the ions in a CsCl-like cell, we can estimate
the cell dimension. Thallium(I) Chloride, TlCl, crystallizes with a CsCl-like
structure. We estimate the the edge length of the unit cell from the published
values of the radii:
The radius of Tl+ is 156 pm
The radius of Cl - is 181 pm
a = d/1.732
= 674 pm/1.732
= 389 pm
Use the links on the right to illustrate the topics listed above.

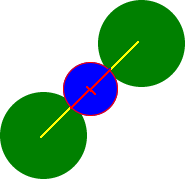
Body
diagonal, d = 674 pm
Thallium(I)
chloride, TlCl, crystallizes with the same structure as CsCl
Cell edge of TlCl ![]() = 457
= 457
Cl -
ion
Cl -
ion
Radii:
181 pm
+ 156 pm
+ 156 pm
+ 181 pm
_______
674 pm
Tl+
ion

Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software