
Each cation in a compound with the CaF2 structure is surrounded by eight anions at the corners of a cube. Each anion is surrounded by a tetrahedron of cations. The coordination number of the cations is eight; that of the anions, four. The coordination geometry of the cations is cubic; that of the anions, tetrahedral.
Click on any cation or anion to highlight the coordination polyhedron. The underlined texts above link to definitions.
Some of the components of the cubes around ions in the drawing are located in adjacent unit cells.
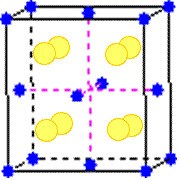























Cation:

Anion:

Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software