
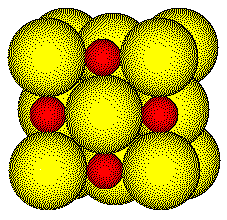
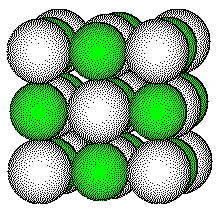
The radius of a Na+ ion is 97 pm; the radius of a Cl - ion is 181 pm; their radius ratio is 0.54. When the ions are drawn to scale, it is difficult to see into the unit cell. Thus we will usually draw the structure with a radius ratio of about 1:1.
Many compounds crystallize with the NaCl structure including all lithium, sodium, and potassium halides and hydrides (with the formula MX) as well as many Group 2 transition metal oxides with the formula MO.
Click the links on the right to illustrate the concepts listed above.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software