
Alternatively,
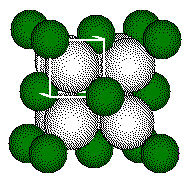
if we know the radii of the ions in a CaF2 -like cell, we can estimate
the cell dimension. We estimate the edge length of the unit cell of CaF2
from published values of the radii:
The radius of Ca2+ is 100 pm.
The radius of F - is 131 pm.
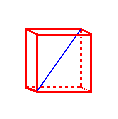
The body diagonal is 462 pm long.
The length of the edge of the small cube is 267 pm, thus the unit cell length is 534 pm. The distance between the center of the fluoride ion and the center of the calcium ion is 1/2 the length of the body diagonal of the small cube or 1/4 the length of the body diagonal of the unit cell.
Click the links on the right to illustrate these concepts.
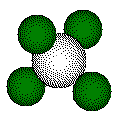
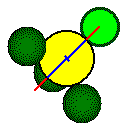
100 pm
+131 pm
+131 pm
+100 pm
-----------
462 pm

by: Journal of Chemical Education Software