
The sodium chloride structure can also be described using an alternate unit cell in a face centered cubic lattice.
Sodium ions are placed on the lattice points.
Chloride ions are placed at the midpoint of each edge and in the center of the alternate cell.
The relationship of the two cells is described in the next two pages.
Click the links on the right to illustrate these topics.
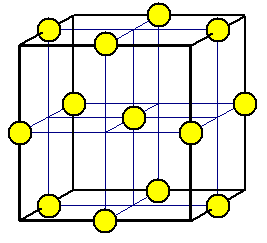
Chloride
ions are placed at the center of the cell and in the center of each edge.

An alternate unit cell is sometimes used to describe the NaCl structure.

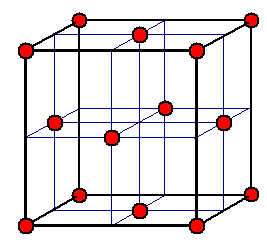
In
the alternate cell, the Na+ ions are placed on the corners
and in the center of each face.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software