
The distance between two atoms is measured by the distance between their nuclei, the internuclear distance.
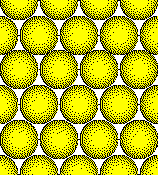
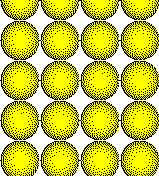
The most efficient packing of atoms in a plane results when the atoms are arranged in a hexagonal array with each atom touching six nearest neighbors. The number of nearest neighbors is called the coordination number. Thus atoms in a closest packed plane have a coordination number of six. A less efficient arrangement is the square array. Each atom has a coordination number of four in the more open square array.
Click the links on the right to illustrate many of the topics that were presented on this page.
 |
 |
Closest Packed Plane
|
Square Packed Plane
|
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software