
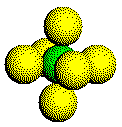
A simple cubic (SC) structure is composed of layers of atoms arranged in a square array. Atoms touch 4 other atoms within the same layer as well as 1 atom in the layer above and 1 atom in the layer below. The six atoms around any given atom form an octahedron.
Click the link on the right to alternate between the coordination sphere and the structure of an SC metal.

Show
Coordination Sphere
Show
Structure
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software