
This is the end of the Body-Centered Cubic Metals section. Click the links on the right to review important concepts.
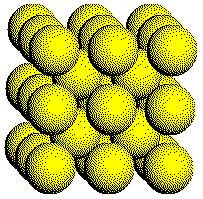
A
portion of the structure of a body-centered cubic metal
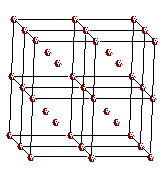
A
body-centered cubic space lattice
Atoms
are located at the lattice points in a body-centered cubic metal

Atoms
in a body-centered cubic metal have a coordination number of eight
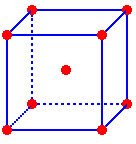
A
body-centered cubic unit cell is a cube with lattice points at the corners
and in the center of the cube


One
atom is located at the center of the unit cell and one-eighth of an atom
is located inside the unit cell at each corner giving a total of two atoms
per unit cell
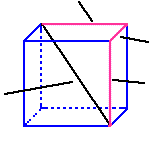
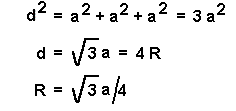
The body diagonal of the cell, d, is four times
R, the radius of the atoms in the unit cell
d = 4/R
![]()
![]()
a, unit cell edge
a, unit cell edge
a, unit cell edge
d, body diagonal
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software