
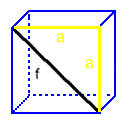
The face diagonal is part of a right triangle that includes two unit cell edges.
f, the length of the face diagonal can be calculated from a, the length of the cell edge using the Pythagorean Theorem.
![]()
The face diagonal is four times the atomic radius (f = 4R) so
![]()
For the FCC metal Pt: a= 392 pm
R = 139 pm
Click the link on the right to view the pythagorean theorem in action. Click the underlined text above to view a definition.
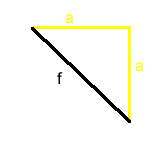
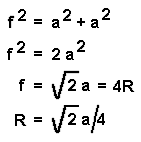
f, the face diagonal
and
a, the unit cell edge
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software