
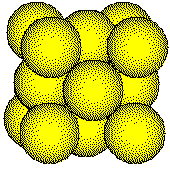
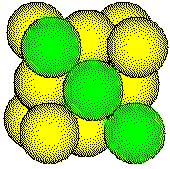
Metal atoms in a face-centered cubic unit cell touch along the face diagonals of the unit cell.
The length of the face diagonal and the radius of the metal atom can be determined by measuring the edge length of the unit cell by x-ray diffraction.
The length of the face diagonal is four times the radius of an atom. Thus the radius of the atom is one-fourth the length of the face diagonal.
Click the links on the right to demonstrate these concepts. The underlined words in the text link to definitions.
Face
Diagonal
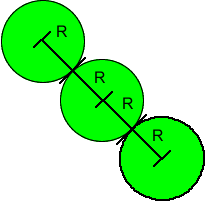
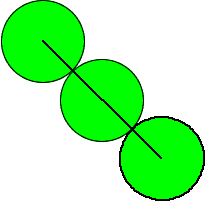
The
face diagonal has length 4R; where R is the radius of the atom
Outline
of three atoms along the face diagonal.
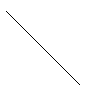
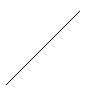





Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software