
Click on the links on the right to review this topic.
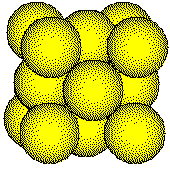
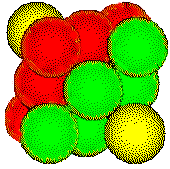
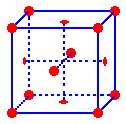
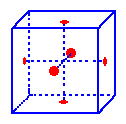
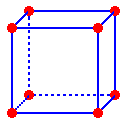
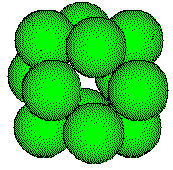

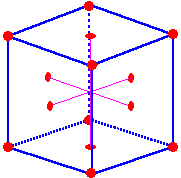
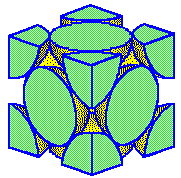
The
atoms in a face-centered cubic unit cell are located on the corners of
a cube and in the centers of the faces
Cubic
close-packed planes pass through a face-centered cubic unit cell
Lattice
points are located on the corners and in the centers of the faces of a face-centered unit
cell
Lattice
points in the faces of a face-centered unit cell
Lattice
points at the corners of a face-centered cubic unit cell
Metal
atoms in a face-centered cubic metal have a coordination number of 12
Lattice
points are located on the corners and in the centers of the faces of a
face-centered cubic unit cell
One
half of an atom is located inside the unit cell in the middle of each face
One
eighth of an atom is located inside the unit cell at each corner
6 faces x 1/2 atoms at each face +
8 corners x 1/8 atom at each corner
= 4 atoms per cell
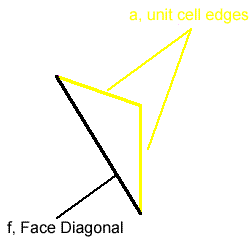
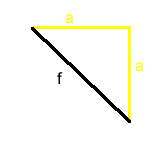
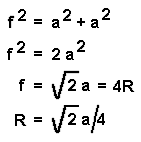
The radius of the atom is given by the equation
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software