
A face-centered cubic unit cell contains part of 1 atom from the 4th layer of a stack of CCP planes;
parts of 6 atoms from the 3rd layer;
parts of 6 atoms from the 2nd layer;
& part of 1 atom from the 1st layer.
Click the links on the right to view the various layers.
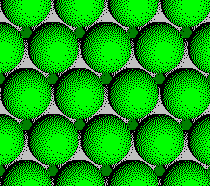
Layer 4 of a CCP Structure
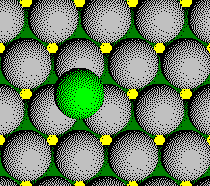
Part of one atom from the 4th layer is in the unit cell.
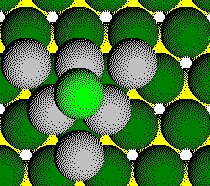
Parts of six atoms from the third layer are in the unit cell.
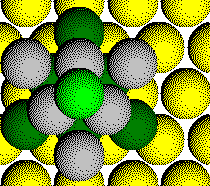
Parts of six atoms from the second layer are in the unit cell
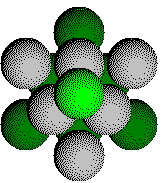
Part of one atom from the first layer is in the unit cell. It is directly below the atom in the fourth layer and is not visible.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software