
The structure of a cubic close-packed metal consists of close-packed planes of atoms in an ..ABC.. orientation. The figure shows the first layer in a CCP structure with an orientation arbitrarily called the A orientation.
Each atom in the structure has the same environment. Thus a lattice point can be placed at the center of each atom. A cubic unit cell can be selected as shown on the next page.
Click the links on the right to view various layers in a CCP structure.
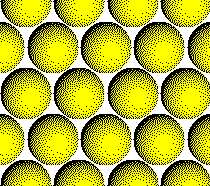
Plane A:
Layer 1 of a CCP Structure
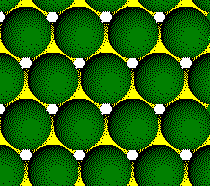
Plane B:
Layer 2 of a CCP Structure
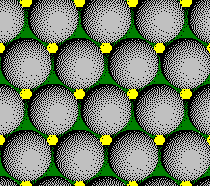
Plane C:
Layer 3 of a CCP Structure
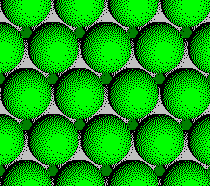
Plane A:
Layer 4 of a CCP Structure
Layer A--
Layer C--
Layer B--
Layer A--
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software