
The coordination number of an atom in a simple cubic structure is 6. The highlighted center atom has a coordination number of 6.
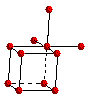
The six nearest neighbors around a metal atom in a simple cubic structure are arranged at the corners of an octahedron. In order to see the octahedral coordination sphere in a simple cubic structure, it is necessary to include atoms on lattice points in adjacent unit cells.
Click the links on the right to illustrate the topics above, and click the underlined text above for definitions.

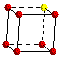
Atoms
in Unit Cell
Unit
Cell of Lattice
Coordination
Sphere
(Octahedron)
Sphere
(Octahedron)
Unit
Cell with
Additional
Lattice Points
Additional
Lattice Points
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software