
If we use x-ray diffraction to find a, the edge length of a CsCl-like unit cell, we can determine ionic sizes.
CsI is isomorphous with CsCl, with a cell edge of 457 pm. We can determine the radius of the iodide ion from the cell dimensions.
d=1.732 x a = 792pm
With d and the published radius of the Cs+ ion, 177 pm, we can estimate the radius of the I - ion.
Radius of I - ion = 219 pm
Use the links on the right to illustrate these concepts. The links above display definitions.
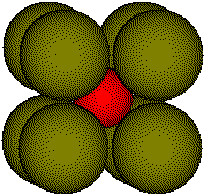
Cesium
iodide, CsI, crystallizes with the same structure as CsCl.
Cell
edge of CsI is 497 pm.
Body
diagonal, d = 792 pm
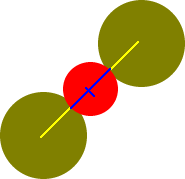
I-
ion
I
- ion
?
+ 177 pm
+ 177 pm
+ ?
_______
Cs+
ion
I-
ion radius calculates to be 219 pm
?
+ 177 pm
+ 177 pm
+ ?
_______
792 pm = d
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software