
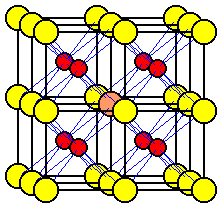
The contents of a unit cell of an ionic compound can be determined by counting the number of ions of each type in the cell. An atom or ion within a cell is not shared with any other unit cells.
An atom or ion at one corner of a unit cell is shared among eight unit cells in the lattice.
Click the links on the right to illustrate the concepts listed above.
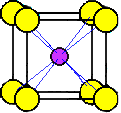
The
cesium ion at the center of this unit cell is not shared with any other
cells.
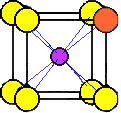
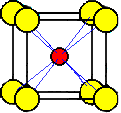
A
chloride ion at the corner of a unit cell is shared by eight unit cells.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software