
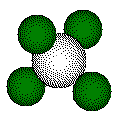
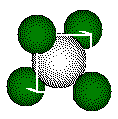
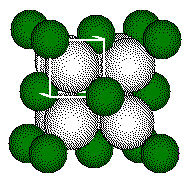
One calculation of distances or radii in a fluorite structure uses the small cube defined by the four calcium ions that form the tetrahedral geometry about the fluoride ion. These calcium ions lie on opposite corners of a cube. The ion inside the tetrahedron lies at the center of the cube.
The edges of the cube containing a tetrahedron in the CaF2 structure is one half the length of the cell edge.
Click the links on the right in order to illustrate these concepts.
The
ions that form this tetrahedron lie on opposite corners of a cube. We
use the cube for calculating distances in the unit cell.
x,
one half of the cell dimension of the CaF2 unit cell.
Cell
dimension of the CaF2 unit cell.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software