
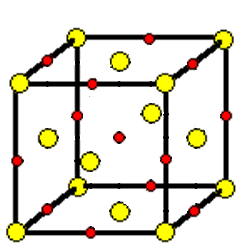
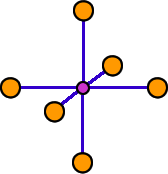
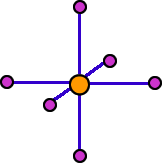
Each cation in a compound with the NaCl structure is surrounded by six anions at the corners of an octahedron. Each anion is surrounded by an octahedron of cations. The coordination number of these ions is six. The coordination geometry is octahedral.
Click on any cation or anion to highlight the coordination polyhedron. Click the links above for definitions.
Some of the components of the octahedra around ions on the edges or faces of a unit cell are located in adjacent unit cells.



























Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software