
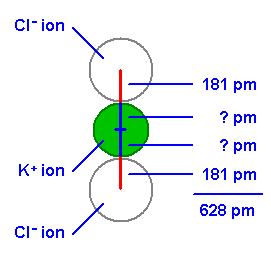
If we use X-ray diffraction to find the edge length of a NaCI-like unit cell and if we know the radius of one ion, we can determine the radius of the other ion in the compound.
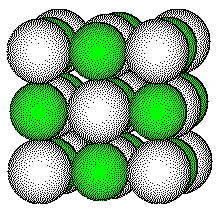
KCl is isomorphous with NaCl, with a cell edge of 628 pm. We can determine the radius of the potassium ion from the cell dimension and the radius of the chloride ion, 181 pm.
Click the links on the right in order to illustrate these concepts. The underlined text above links to definitions.
KCl crystallizes with the same structures as NaCl

The
radius of the potasium ion is 133 pm.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software