
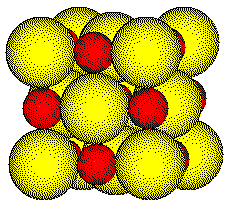
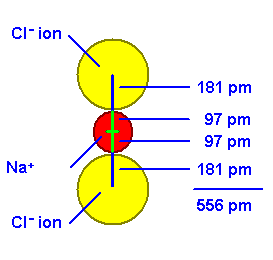
If we know the ionic radii of the ions that crystallize in a NaCl type unit cell, we can calculate the length of the unit cell edge.
The radius of a sodium ion is 97 picometers (pm). The radius of a chloride ion is 181 pm.
The sum of the four radii lying along the edge is 556 pm.
Click the links on the right to illustrate these concepts.
The underlined text above links to a definition.
![]()
Unit Cell Edge
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software