
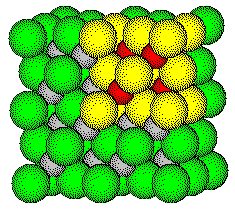
Zinc blende, the cubic form of zinc sulfide, crystallizes with a face centered cubic space lattice.
One description of the structure places the centers of sulfide ions
at the lattice
points.
The zinc ions occupy one-half of the tetrahedral holes in the anion array.
The ZnS unit cell contains several zinc ions and sulfide ions.
Click the links on the right to illustrate these concepts.
The underlined texts above link to definitions.
Zinc blende, a cubic form of zinc sulfide, crystallizes
with a face centered cubic lattice.
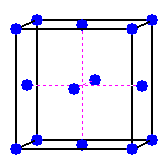
The Face Centered Cubic Cell
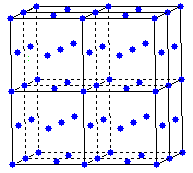
Sulfide ions are located on lattice points
and form a face centered cubic array.
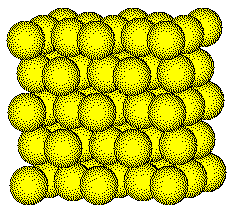
Zinc ions occupy one-half of the tetrahedral
holes in the face centered cubic array of sulfide ions.
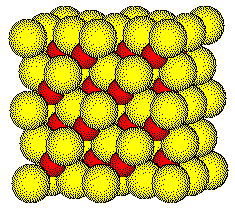
A
ZnS Unit Cell
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software