
The unit cells of zinc blende and of structures isomorphous with zinc blende are face centered cubic. Lattice points are located on the corners and in the centers of the faces of a cubic unit cell.
One common description of the zinc blende unit cell places sulfide ions on the lattice points.
Zinc ions are located in one-half of the tetrahedral holes within the unit cell.
Click the links on the right to illustrate the above topics. The underlined text above links to a definition.
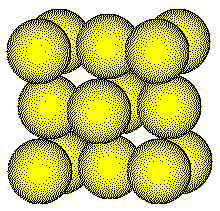
Zinc blende crystallizes with a face centered
cubic unit cell.

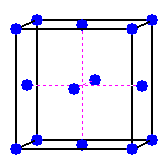
Sulfide ions are located on the lattice points
at the eight corners of the unit cell and in the center of each face.

Zinc ions are located in one
half of the tetrahedral holes in the unit cell.
Zinc blende crystallizes with a face centered
cubic unit cell.
Lattice points are located on the corners and in the center of the faces of the unit cell.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software